Is N2o4 An Ionic Compound
| |||
 Nitrogen dioxide at −196 °C, 0 °C, 23 °C, 35 °C, and 50 °C. (NO | |||
| Names | |||
|---|---|---|---|
| IUPAC name Dinitrogen tetraoxide | |||
| Identifiers | |||
| CAS Number |
| ||
| 3D model (JSmol) |
| ||
| ChEBI |
| ||
| ChemSpider |
| ||
| ECHA InfoCard | 100.031.012 | ||
| EC Number |
| ||
| Gmelin Reference | 2249 | ||
| PubChem CID |
| ||
| RTECS number |
| ||
| UNII |
| ||
| Un number | 1067 | ||
| CompTox Dashboard (EPA) |
| ||
| InChI
| |||
| SMILES
| |||
| Properties | |||
| Chemic formula | N2O4 | ||
| Molar mass | 92.011g/mol | ||
| Appearance | Colourless liquid, orange gas | ||
| Density | 1.44246g/cmiii (liquid, 21 °C) | ||
| Melting point | −xi.ii °C (11.8 °F; 261.9 G) and decomposes to NO2 | ||
| Boiling point | 21.69 °C (71.04 °F; 294.84 Thou) | ||
| Solubility in water | Reacts to class nitrous and nitric acids | ||
| Vapor force per unit area | 96kPa (20°C)[1] | ||
| Magnetic susceptibility (χ) | −23.0·10−half dozen cm3/mol | ||
| Refractive index (northward D) | 1.00112 | ||
| Structure | |||
| Molecular shape | Planar, D 2h | ||
| Dipole moment | pocket-size, non-zero | ||
| Thermochemistry | |||
| Std molar | 304.29J/K⋅mol[2] | ||
| Std enthalpy of | +nine.16kJ/mol[2] | ||
| Hazards | |||
| GHS labelling: | |||
| Pictograms |      | ||
| Signal discussion | Danger | ||
| Hazard statements | H270, H280, H314, H330, H335, H336 | ||
| Precautionary statements | P220, P244, P260, P261, P264, P271, P280, P284, P301+P330+P331, P303+P361+P353, P304+P340, P305+P351+P338, P310, P312, P320, P321, P363, P370+P376, P403, P403+P233, P405, P410+P403, P501 | ||
| NFPA 704 (fire diamond) | [3] [4] 3 0 0 OX | ||
| Wink betoken | Non-flammable | ||
| Safety data sheet (SDS) | External MSDS | ||
| Related compounds | |||
| Related nitrogen oxides |
| ||
| Except where otherwise noted, data are given for materials in their standard country (at 25 °C [77 °F], 100 kPa). Infobox references | |||
Dinitrogen tetroxide, commonly referred to as nitrogen tetroxide (NTO), and occasionally (commonly among ex-USSR/Russia rocket engineers) equally amyl, is the chemical compound N2O4. It is a useful reagent in chemical synthesis. It forms an equilibrium mixture with nitrogen dioxide. Its molar mass is 92.011 g/mol.
Dinitrogen tetroxide is a powerful oxidizer that is hypergolic (spontaneously reacts) upon contact with various forms of hydrazine, which has fabricated the pair a mutual bipropellant for rockets.
Structure and properties [edit]
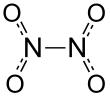
Dinitrogen tetroxide could exist regarded equally 2 nitro groups (-NO2) bonded together. It forms an equilibrium mixture with nitrogen dioxide.[five] The molecule is planar with an N-Northward bond distance of 1.78Å and N-O distances of 1.19Å. The Northward-N distance corresponds to a weak bail, since it is significantly longer than the boilerplate N-Due north single bond length of i.45Å.[6] This exceptionally weak σ bail (amounting to overlapping of the sp 2 hybrid orbitals of the two NO2 units[vii]) results from the simultaneous delocalization of the bonding electron pair across the whole Due northiiOiv molecule, and the considerable electrostatic repulsion of the doubly occupied molecular orbitals of each NO2 unit.[8]
Dissimilar NO2, N2O4 is diamagnetic since it has no unpaired electrons.[9] The liquid is too colorless but tin can appear as a brownish yellow liquid due to the presence of NO2 according to the following equilibrium:
- NiiOiv ⇌ two NO2
Higher temperatures push the equilibrium towards nitrogen dioxide. Inevitably, some dinitrogen tetroxide is a component of smog containing nitrogen dioxide.
Production [edit]
Nitrogen tetroxide is made by the catalytic oxidation of ammonia: steam is used as a diluent to reduce the combustion temperature. In the beginning step, the ammonia is oxidized into nitric oxide:
- iv NH3 + five O2 → 4 NO + 6 H2O
Most of the water is condensed out, and the gases are further cooled; the nitric oxide that was produced is oxidized to nitrogen dioxide, which is and then dimerized into nitrogen tetroxide:
- 2 NO + Otwo → 2 NO2
- 2 NO2 ⇌ NiiO4
and the residual of the water is removed every bit nitric acid. The gas is essentially pure nitrogen dioxide, which is condensed into dinitrogen tetroxide in a alkali-cooled liquefier.[ commendation needed ]
Dinitrogen tetroxide can also exist made through the reaction of full-bodied nitric acid and metallic copper. This synthesis is more applied in a laboratory setting and is normally[ when? ] used[ by whom? ] as a demonstration or experiment in undergraduate chemical science labs.[ commendation needed ] The oxidation of copper past nitric acrid is a circuitous reaction forming various nitrogen oxides of varying stability which depends on the concentration of the nitric acrid, presence of oxygen, and other factors. The unstable species farther react to form nitrogen dioxide which is and so purified and condensed to form dinitrogen tetroxide.
Use as a rocket propellant [edit]
Nitrogen tetroxide is used equally an oxidizing agent in 1 of the most of import rocket propellants because it can exist stored as a liquid at room temperature. Pedro Paulet, a Peruvian polymath, reported in 1927 that he had experimented in the 1890s with a rocket engine that used spring-loaded nozzles that periodically introduced vaporized nitrogen tetroxide and a petroleum benzine to a spark plug for ignition, with the engine putting out 300 pulsating explosions per minute.[10] [11] Paulet would become on to visit the German rocket association Verein für Raumschiffahrt (VfR) and on March 15, 1928, Valier applauded Paulet's liquid-propelled rocket pattern in the VfR publication Die Rakete, maxim the engine had "amazing power".[12] Paulet would soon be approached past Nazi Federal republic of germany to help develop rocket technology, though he refused to assistance and never shared the formula for his propellant.[13]
In early 1944, inquiry on the usability of dinitrogen tetroxide equally an oxidizing agent for rocket fuel was conducted by German scientists, although the Germans only used it to a very limited extent as an condiment for Due south-Stoff (fuming nitric acrid). It became the storable oxidizer of selection for many rockets in both the United States and USSR past the late 1950s. Information technology is a hypergolic propellant in combination with a hydrazine-based rocket fuel. Ane of the earliest uses of this combination was on the Titan family of rockets used originally as ICBMs and so every bit launch vehicles for many spacecraft. Used on the U.South. Gemini and Apollo spacecraft and also on the Infinite Shuttle, it continues to be used as station-keeping propellant on almost geo-stationary satellites, and many deep-space probes. It is too the primary oxidizer for Russian federation's Proton rocket.
When used as a propellant, dinitrogen tetroxide is usually referred to only equally nitrogen tetroxide and the abbreviation NTO is extensively used. Additionally, NTO is often used with the addition of a small per centum of nitric oxide, which inhibits stress-corrosion peachy of titanium alloys, and in this grade, propellant-grade NTO is referred to as mixed oxides of nitrogen (Mon). Virtually spacecraft now use MON instead of NTO; for case, the Space Shuttle reaction control system used MON3 (NTO containing 3% NO by weight).[14]
The Apollo-Soyuz mishap [edit]
On 24 July 1975, NTO poisoning afflicted three U.S. astronauts on the final descent to Earth later on the Apollo-Soyuz Test Projection flight. This was due to a switch accidentally left in the wrong position, which allowed the mental attitude control thrusters to fire after the motel fresh air intake was opened, allowing NTO fumes to enter the cabin. One crew fellow member lost consciousness during descent. Upon landing, the crew was hospitalized for v days for chemical-induced pneumonia and edema.[fifteen] [16]
Ability generation using Northward2Ofour [edit]
The tendency of NtwoOiv to reversibly break into NO2 has led to inquiry into its use in avant-garde power generation systems as a so-called dissociating gas.[17] "Cool" dinitrogen tetroxide is compressed and heated, causing it to dissociate into nitrogen dioxide at half the molecular weight. This hot nitrogen dioxide is expanded through a turbine, cooling it and lowering the pressure, then cooled further in a estrus sink, causing information technology to recombine into nitrogen tetroxide at the original molecular weight. It is then much easier to compress to commencement the entire cycle again. Such dissociative gas Brayton cycles have the potential to considerably increment efficiencies of power conversion equipment.[18]
Chemical reactions [edit]
Intermediate in the manufacture of nitric acrid [edit]
Nitric acrid is manufactured on a large scale via North2O4. This species reacts with water to give both nitrous acid and nitric acrid:
- N2O4 + HtwoO → HNO2 + HNO3
The coproduct HNO2 upon heating disproportionates to NO and more nitric acid. When exposed to oxygen, NO is converted back into nitrogen dioxide:
- ii NO + O2 → 2 NOii
The resulting NO2 and Due north2O4 tin can be returned to the cycle to requite the mixture of nitrous and nitric acids again.
Synthesis of metal nitrates [edit]
N2O4 undergoes molecular autoionization to give [NO+] [NO3 −], with the former nitrosonium ion existence a strong oxidant. Various anhydrous transition metal nitrate complexs can exist prepared from N2O4 and base metal.[nineteen]
- 2 N2O4 + G → ii NO + M(NOthree)two
where Chiliad = Cu, Zn, or Sn.
If metal nitrates are prepared from NorthwardiiO4 in completely anhydrous conditions, a range of covalent metal nitrates can exist formed with many transition metals. This is because in that location is a thermodynamic preference for the nitrate ion to bond covalently with such metals rather than grade an ionic structure. Such compounds must be prepared in anhydrous atmospheric condition, since the nitrate ion is a much weaker ligand than h2o, and if h2o is present the simple hydrated nitrate volition form. The anhydrous nitrates concerned are themselves covalent, and many, due east.g. anhydrous copper nitrate, are volatile at room temperature. Anhydrous titanium nitrate sublimes in vacuum at just xl °C. Many of the anhydrous transition element nitrates have hit colours. This co-operative of chemistry was developed past Cliff Addison and Norman Logan at the University of Nottingham in the UK during the 1960s and 1970s when highly efficient desiccants and dry boxes started to become available.
References [edit]
- ^ International Chemical Safety Card https://world wide web.ilo.org/dyn/icsc/showcard.display?p_lang=en&p_card_id=0930&p_version=2
- ^ a b P.W. Atkins and J. de Paula, Physical Chemical science (8th ed., Due west.H. Freeman, 2006) p.999
- ^ "Chemical Datasheet: Nitrogen tetroxide". CAMEO Chemicals NOAA . Retrieved 8 September 2020.
- ^ "Compound Summary: Dinitrogen tetroxide". PubChem . Retrieved viii September 2020.
- ^ Bent, Henry A. (1963). "Dimers of Nitrogen Dioxide. II. Structure and Bonding". Inorganic Chemistry. 2 (iv): 747–752. doi:10.1021/ic50008a020.
- ^ Petrucci, Ralph H.; Harwood, William S.; Herring, F. Geoffrey (2002). General chemistry: principles and modern applications (8th ed.). Upper Saddle River, N.J: Prentice Hall. p. 420. ISBN978-0-13-014329-seven. LCCN 2001032331. OCLC 46872308.
- ^ Rayner-canham, Geoff (2013). Descriptive inorganic chemical science (6th ed.). p. 400. ISBN978-one-319-15411-0. OCLC 1026755795.
- ^ Ahlrichs, Reinhart; Keil, Frerich (1974-12-01). "Construction and bonding in dinitrogen tetroxide (N2O4)". Journal of the American Chemical Gild. 96 (25): 7615–7620. doi:ten.1021/ja00832a002. ISSN 0002-7863.
- ^ Holleman, A. F.; Wiberg, E. "Inorganic Chemistry" Academic Printing: San Diego, 2001. ISBN 978-0-12-352651-9.
- ^ Gonzales Obando, Diana (2021-07-22). "Pedro Paulet: el genio peruano que se adelantó a su época y fundó la era espacial". El Comercio (in Castilian). Retrieved 2022-03-thirteen .
- ^ "Un peruano Pedro Paulet reclama la propiedad de su invento". El Comercio (in Spanish). 25 August 1927. Retrieved 2022-03-xiii .
- ^ Mejía, Álvaro (2017). Pedro Paulet, sabio multidisciplinario (in Spanish). Universidad Católica San Pablo. pp. 95–122.
- ^ "El peruano que se convirtió en el padre de la astronáutica inspirado por Julio Verne y que aparece en los nuevos billetes de 100 soles". BBC News (in Spanish). Retrieved 2022-03-xi .
- ^ "Rocket Propellant Index". Archived from the original on 2008-05-xi. Retrieved 2005-03-01 .
- ^ "Make Takes Blame For Apollo Gas Leak", Florence, AL - Times Daily newspaper, Aug. 10, 1975
- ^ Sotos, John G., Physician. "Astronaut and Cosmonaut Medical Histories", May 12, 2008, accessed April ane, 2011.
- ^ Stochl, Robert J. (1979). Potential performance comeback past using a reacting gas (nitrogen tetroxide) as the working fluid in a closed Brayton cycle (PDF) (Technical report). NASA. TM-79322.
- ^ Ragheb, R. "Nuclear Reactors Concepts and Thermodynamic Cycles" (PDF) . Retrieved 1 May 2013.
- ^ Addison, C. Clifford (Feb 1980). "Dinitrogen tetroxide, nitric acid, and their mixtures as media for inorganic reactions". Chemical Reviews. 80 (1): 21–39. doi:ten.1021/cr60323a002.
External links [edit]
- International Chemical Safety Card 0930
- National Pollutant Inventory – Oxides of nitrogen fact sheet
- NIOSH Pocket Guide to Chemical Hazards: Nitrogen tetroxide
- Air Liquide Gas Encyclopedia: NOii / Due north2O4
- Poliakoff, Martyn (2009). "The Chemical science of Lunar Lift-Off: Our Apollo eleven 40th Anniversary Special". The Periodic Table of Videos. Academy of Nottingham.
Is N2o4 An Ionic Compound,
Source: https://en.wikipedia.org/wiki/Dinitrogen_tetroxide
Posted by: pooreexagavied.blogspot.com




0 Response to "Is N2o4 An Ionic Compound"
Post a Comment