A Semimetal In Group 5a
18.seven: The Group 5A Elements
- Page ID
- 15212
- To sympathise the trends in backdrop and reactivity of the group fifteen elements: the pnicogens.
Like the group 14 elements, the lightest fellow member of group 15, nitrogen, is found in nature as the free chemical element, and the heaviest elements have been known for centuries considering they are easily isolated from their ores. Antimony (Sb) was probably the showtime of the pnicogens to be obtained in elemental course and recognized as an element. Its atomic symbol comes from its Roman name: stibium. It is found in stibnite (SbiiSouththree), a blackness mineral that has been used every bit a cosmetic (an early on form of mascara) since biblical times, and it is hands reduced to the metal in a charcoal fire (Figure \(\PageIndex{i}\)). The Egyptians used antimony to glaze copper objects equally early equally the third millennium BC, and antimony is still used in alloys to meliorate the tonal quality of bells.
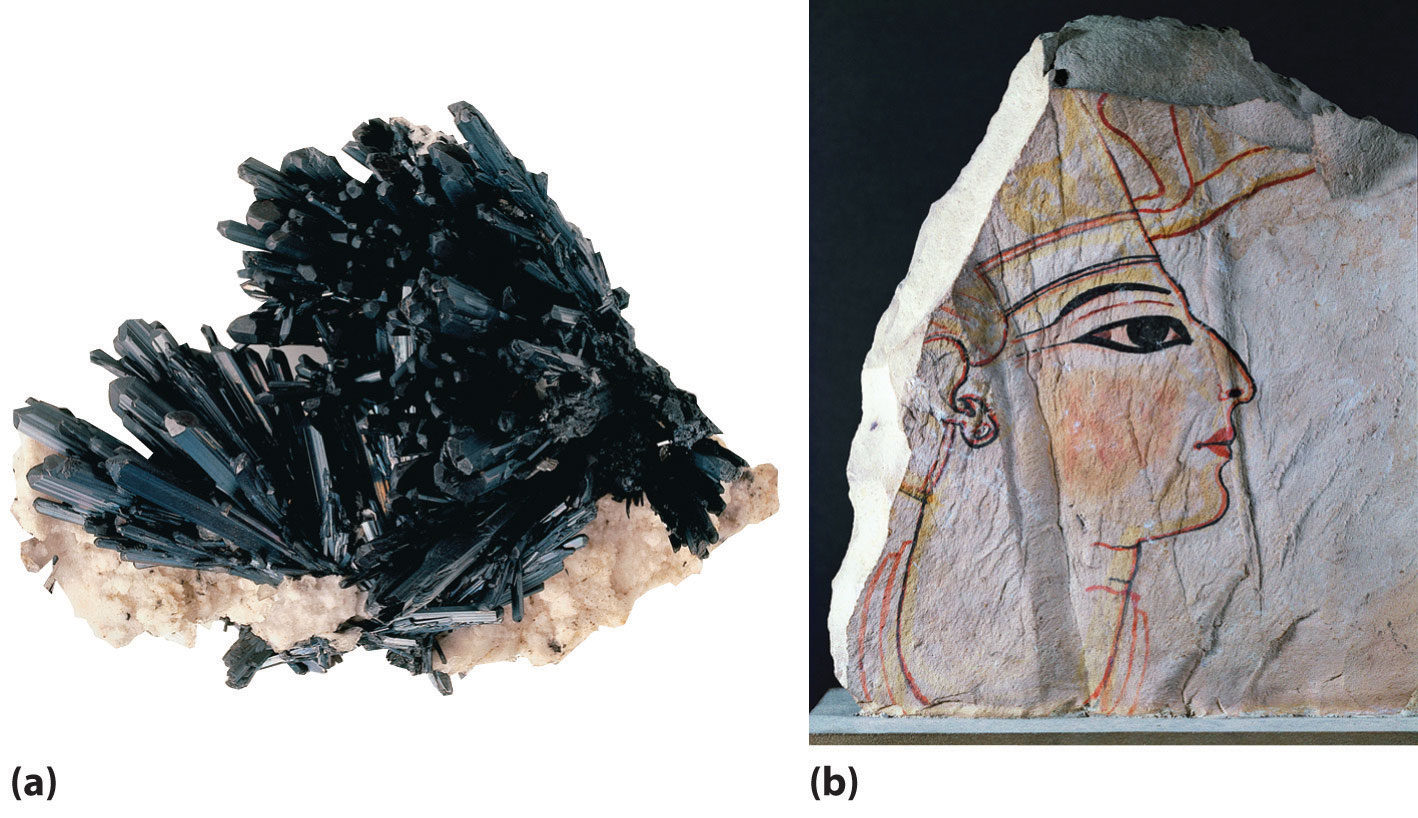
In the grade of its yellow sulfide ore, orpiment (Equally2Siii), arsenic (Equally) has been known to physicians and professional assassins since ancient Greece, although elemental arsenic was not isolated until centuries later. The history of bismuth (Bi), in contrast, is more difficult to follow because early on alchemists often confused information technology with other metals, such as lead, tin, antimony, and even silver (due to its slightly pinkish-white luster). Its proper name comes from the old German language wismut, pregnant "white metal." Bismuth was finally isolated in the 15th century, and it was used to make movable type for press before long after the invention of the Gutenberg printing process in 1440. Bismuth is used in printing because it is one of the few substances known whose solid country is less dense than the liquid. Consequently, its alloys expand as they cool, filling a mold completely and producing crisp, articulate letters for typesetting.
Phosphorus was discovered in 1669 past the German alchemist Hennig Brandt, who was looking for the "philosophers' stone," a mythical substance capable of converting base metals to silver or gold. Believing that human urine was the source of the key ingredient, Brandt obtained several dozen buckets of urine, which he allowed to putrefy. The urine was distilled to dryness at high temperature and then condensed; the last fumes were nerveless under h2o, giving a waxy white solid that had unusual properties. For instance, it glowed in the dark and burst into flames when removed from the water. (Unfortunately for Brandt, notwithstanding, information technology did non turn lead into gilt.) The element was given its current proper name (from the Greek phos, significant "light," and phoros, pregnant "bringing") in the 17th century. For more than than a century, the only mode to obtain phosphorus was the distillation of urine, but in 1769 it was discovered that phosphorus could be obtained more easily from basic. During the 19th century, the demand for phosphorus for matches was so great that battlefields and paupers' graveyards were systematically scavenged for basic. Early matches were pieces of wood coated with elemental phosphorus that were stored in an evacuated glass tube and ignited when the tube was broken (which could crusade unfortunate accidents if the matches were kept in a pocket!).
Unfortunately, elemental phosphorus is volatile and highly toxic. Information technology is captivated by the teeth and destroys os in the jaw, leading to a painful and fatal condition called "phossy jaw," which for many years was accepted every bit an occupational hazard of working in the match industry.
Although nitrogen is the almost abundant element in the atmosphere, it was the last of the pnicogens to exist obtained in pure form. In 1772, Daniel Rutherford, working with Joseph Blackness (who discovered COtwo), noticed that a gas remained when COtwo was removed from a combustion reaction. Antoine Lavoisier called the gas azote, meaning "no life," considering it did non back up life. When information technology was discovered that the same element was also present in nitric acid and nitrate salts such as KNO3 (nitre), it was named nitrogen. About 90% of the nitrogen produced today is used to provide an inert atmosphere for processes or reactions that are oxygen sensitive, such as the production of steel, petroleum refining, and the packaging of foods and pharmaceuticals.
Training and General Properties of the Group 15 Elements
Because the atmosphere contains several trillion tons of elemental nitrogen with a purity of about lxxx%, it is a huge source of nitrogen gas. Distillation of liquefied air yields nitrogen gas that is more 99.99% pure, but small amounts of very pure nitrogen gas tin exist obtained from the thermal decomposition of sodium azide:
\[\mathrm{2NaN_3(s)\xrightarrow{\Delta}2Na(l)+3N_2(g)} \label{Eq1}\]
In contrast, Globe'south crust is relatively poor in nitrogen. The but important nitrogen ores are large deposits of KNOthree and NaNO3 in the deserts of Republic of chile and Russia, which were apparently formed when aboriginal alkaline lakes evaporated. Consequently, virtually all nitrogen compounds produced on an industrial calibration use atmospheric nitrogen as the starting fabric. Phosphorus, which constitutes but about 0.1% of Globe's chaff, is much more abundant in ores than nitrogen. Like aluminum and silicon, phosphorus is always constitute in combination with oxygen, and large inputs of free energy are required to isolate it.
The other 3 pnicogens are much less abundant: arsenic is plant in World'south crust at a concentration of about ii ppm, antimony is an lodge of magnitude less abundant, and bismuth is almost as rare every bit aureate. All iii elements have a high affinity for the chalcogens and are normally plant as the sulfide ores (M2Siii), often in combination with sulfides of other heavy elements, such equally copper, silver, and pb. Hence a major source of antimony and bismuth is flue dust obtained by smelting the sulfide ores of the more abundant metals.
In group 15, as elsewhere in the p cake, we run across large differences between the lightest element (N) and its congeners in size, ionization energy, electron affinity, and electronegativity (Table \(\PageIndex{1}\)). The chemical behavior of the elements can be summarized rather simply: nitrogen and phosphorus behave chemically like nonmetals, arsenic and antimony conduct like semimetals, and bismuth behaves like a metal. With their ns2npthree valence electron configurations, all form compounds by losing either the three np valence electrons to grade the +3 oxidation state or the iii np and the two ns valence electrons to give the +5 oxidation state, whose stability decreases smoothly from phosphorus to bismuth. In addition, the relatively large magnitude of the electron affinity of the lighter pnicogens enables them to form compounds in the −3 oxidation land (such as NHthree and PHthree), in which three electrons are formally added to the neutral atom to give a filled np subshell. Nitrogen has the unusual power to form compounds in nine different oxidation states, including −three, +iii, and +5. Because neutral covalent compounds of the trivalent pnicogens contain a lone pair of electrons on the key atom, they tend to carry as Lewis bases.
| Property | Nitrogen | Phosphorus | Arsenic | Antimony | Bismuth |
|---|---|---|---|---|---|
| *The configuration shown does not include filled d and f subshells. †For white phosphorus. ‡For gray arsenic. §The values cited are for half-dozen-coordinate ions in the indicated oxidation states. The N5 +, P5 +, and As5 + ions are not known species. ||The chemical form of the elements in these oxidation states varies considerably. For Northward, the reaction is NO3 − + 3H+ + 2e− → HNO2 + H2O; for P and As, information technology is \(\ce{H3EO4 + 2H^{+} + 2e^{−} → H3EO3 + H2O}\); and for Sb it is \(\ce{Sb2O5 + 4e^{-} + 10H^{+} → 2Sb^{3+} + 5H2O}\). | |||||
| atomic symbol | N | P | As | Sb | Bi |
| diminutive number | vii | 15 | 33 | 51 | 83 |
| atomic mass (amu) | 14.01 | xxx.97 | 74.92 | 121.76 | 209.98 |
| valence electron configuration* | 2s22piii | 3sii3p3 | 4s24pthree | 5s25p3 | 6s26p3 |
| melting point/boiling signal (°C) | −210/−196 | 44.15/281c | 817 (at 3.seventy MPa)/603 (sublimes)† | 631/1587 | 271/1564 |
| density (grand/cmiii) at 25°C | 1.xv (g/50) | one.82† | 5.75‡ | 6.68 | ix.79 |
| atomic radius (pm) | 56 | 98 | 114 | 133 | 143 |
| outset ionization energy (kJ/mol) | 1402 | 1012 | 945 | 831 | 703 |
| common oxidation land(s) | −3 to +five | +v, +3, −3 | +5, +3 | +5, +iii | +3 |
| ionic radius (pm)§ | 146 (−three), 16 (+3) | 212 (−3), 44 (+three) | 58 (+3) | 76 (+3), 60 (+5) | 103 (+3) |
| electron affinity (kJ/mol) | 0 | −72 | −78 | −101 | −91 |
| electronegativity | 3.0 | ii.2 | two.2 | ii.ane | 1.9 |
| standard reduction potential (E°, 5) (Due eastV → Eastward3 in acidic solution)|| | +0.93 | −0.28 | +0.56 | +0.65 | — |
| production of reaction with O2 | NO2, NO | PivO6, PfourO10 | As4O6 | Sb2O5 | Bi2O3 |
| type of oxide | acidic (NO2), neutral (NO, NtwoO) | acidic | acidic | amphoteric | basic |
| production of reaction with N2 | — | none | none | none | none |
| product of reaction with X2 | none | PXthree, PX5 | AsF5, AsX3 | SbF5, SbClv, SbBrthree, SbIthree | BiF5, BiX3 |
| production of reaction with H2 | none | none | none | none | none |
In grouping 15, the stability of the +5 oxidation state decreases from P to Bi.
Because neutral covalent compounds of the trivalent group 15 elements have a lone pair of electrons on the central atom, they tend to exist Lewis bases.
Reactions and Compounds of Nitrogen
Like carbon, nitrogen has four valence orbitals (one 2s and three 2p), so information technology can participate in at most 4 electron-pair bonds by using sp3 hybrid orbitals. Unlike carbon, however, nitrogen does not form long bondage considering of repulsive interactions between lone pairs of electrons on adjacent atoms. These interactions go important at the shorter internuclear distances encountered with the smaller, 2nd-period elements of groups 15, 16, and 17. Stable compounds with N–N bonds are express to bondage of no more than 3 N atoms, such as the azide ion (N3 −).
Nitrogen is the only pnicogen that usually forms multiple bonds with itself and other 2d-flow elements, using π overlap of adjacent np orbitals. Thus the stable form of elemental nitrogen is Northwardtwo, whose Northward≡North bail is so strong (DNorth≡N = 942 kJ/mol) compared with the North–N and N=N bonds (DN–N = 167 kJ/mol; DN =N = 418 kJ/mol) that all compounds containing N–North and N=N bonds are thermodynamically unstable with respect to the formation of N2. In fact, the formation of the N≡Northward bond is so thermodynamically favored that virtually all compounds containing N–N bonds are potentially explosive.
Again in contrast to carbon, nitrogen undergoes just two important chemical reactions at room temperature: it reacts with metallic lithium to form lithium nitride, and it is reduced to ammonia past certain microorganisms. At college temperatures, nonetheless, N2 reacts with more than electropositive elements, such as those in grouping xiii, to give binary nitrides, which range from covalent to ionic in grapheme. Similar the corresponding compounds of carbon, binary compounds of nitrogen with oxygen, hydrogen, or other nonmetals are ordinarily covalent molecular substances.
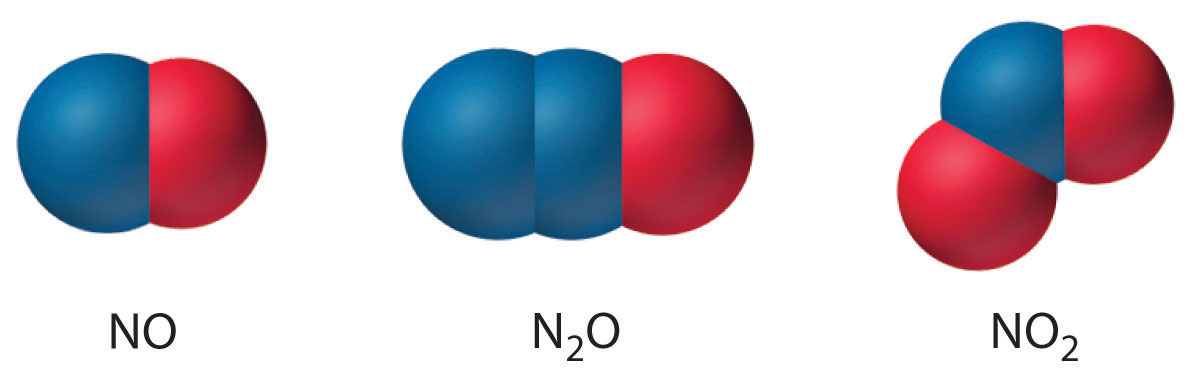
Few binary molecular compounds of nitrogen are formed by direct reaction of the elements. At elevated temperatures, N2 reacts with H2 to course ammonia, with O2 to grade a mixture of NO and NOii, and with carbon to form cyanogen (Due north≡C–C≡N); elemental nitrogen does not react with the halogens or the other chalcogens. Nonetheless, all the binary nitrogen halides (NXiii) are known. Except for NFthree, all are toxic, thermodynamically unstable, and potentially explosive, and all are prepared by reacting the halogen with NHthree rather than Ntwo. Both nitrogen monoxide (NO) and nitrogen dioxide (NO2) are thermodynamically unstable, with positive free energies of formation. Different NO, NO2 reacts readily with excess water, forming a 1:1 mixture of nitrous acrid (HNOtwo) and nitric acid (HNO3):
\[\ce{2NO2(g) + H2O(l) \rightarrow HNO2(aq) + HNO3(aq)} \label{Eq2}\]
Nitrogen besides forms Due northtwoO (dinitrogen monoxide, or nitrous oxide), a linear molecule that is isoelectronic with COii and tin can be represented as −North=N+=O. Like the other two oxides of nitrogen, nitrous oxide is thermodynamically unstable. The structures of the three common oxides of nitrogen are as follows:
Few binary molecular compounds of nitrogen are formed by the direct reaction of the elements.
At elevated temperatures, nitrogen reacts with highly electropositive metals to grade ionic nitrides, such as Li3Northward and CathreeN2. These compounds consist of ionic lattices formed past Gn + and Due norththree− ions. Just as boron forms interstitial borides and carbon forms interstitial carbides, with less electropositive metals nitrogen forms a range of interstitial nitrides, in which nitrogen occupies holes in a close-packed metallic structure. Like the interstitial carbides and borides, these substances are typically very hard, high-melting materials that have metallic luster and electrical conductivity.
Nitrogen also reacts with semimetals at very high temperatures to produce covalent nitrides, such as Si3Northward4 and BN, which are solids with extended covalent network structures similar to those of graphite or diamond. Consequently, they are usually loftier melting and chemically inert materials.
Ammonia (NH3) is one of the few thermodynamically stable binary compounds of nitrogen with a nonmetal. It is not combustible in air, but it burns in an Oii atmosphere:
\[\ce{4NH3(g) + 3O2(chiliad) \rightarrow 2N2(g) + 6H2O(thou)} \label{Eq3}\]
About ten% of the ammonia produced annually is used to brand fibers and plastics that incorporate amide bonds, such as nylons and polyurethanes, while five% is used in explosives, such every bit ammonium nitrate, TNT (trinitrotoluene), and nitroglycerine. Large amounts of anhydrous liquid ammonia are used every bit fertilizer.
Nitrogen forms two other important binary compounds with hydrogen. Hydrazoic acid (HN3), also called hydrogen azide, is a colorless, highly toxic, and explosive substance. Hydrazine (N2Hiv) is also potentially explosive; it is used as a rocket propellant and to inhibit corrosion in boilers.
B, C, and N all react with transition metals to grade interstitial compounds that are hard, high-melting materials.
For each reaction, explain why the given products form when the reactants are heated.
- Sr(southward) + NiiO(g) \(\xrightarrow{\Delta}\) SrO(s) + Ntwo(grand)
- NHivNOii(s) \(\xrightarrow{\Delta}\) N2(g) + 2H2O(g)
- Lead(NO3)two(southward) \(\xrightarrow{\Delta}\) PbO2(s) + 2NO2(grand)
Given: balanced chemical equations
Asked for: why the given products form
Strategy:
Classify the blazon of reaction. Using periodic trends in atomic properties, thermodynamics, and kinetics, explain why the observed reaction products form.
Solution
- As an alkali metallic, strontium is a stiff reductant. If the other reactant can act every bit an oxidant, then a redox reaction will occur. Nitrous oxide contains nitrogen in a low oxidation land (+1), so nosotros would non ordinarily consider it an oxidant. Nitrous oxide is, all the same, thermodynamically unstable (ΔH°f > 0 and ΔG°f > 0), and it tin be reduced to N2, which is a stable species. Consequently, we predict that a redox reaction will occur.
- When a substance is heated, a decomposition reaction probably will occur, which often involves the release of stable gases. In this case, ammonium nitrite contains nitrogen in two different oxidation states (−3 and +iii), so an internal redox reaction is a possibility. Due to its thermodynamic stability, Northward2 is the probable nitrogen-containing product, whereas we predict that H and O volition combine to course H2O.
- Over again, this is probably a thermal decomposition reaction. If ane element is in an normally high oxidation state and another in a low oxidation state, a redox reaction will probably occur. Pb nitrate contains the Pbtwo + cation and the nitrate anion, which contains nitrogen in its highest possible oxidation country (+5). Hence nitrogen can be reduced, and we know that lead tin be oxidized to the +4 oxidation state. Consequently, it is likely that atomic number 82(Ii) nitrate will decompose to pb(Four) oxide and nitrogen dioxide when heated. Even though PbO2 is a powerful oxidant, the release of a gas such as NO2 tin frequently drive an otherwise unfavorable reaction to completion (Le Chatelier's principle). Note, however, that PbO2 will probably decompose to PbO at high temperatures.
Predict the product(due south) of each reaction and write a counterbalanced chemical equation for each reaction.
- NO(one thousand) + HtwoO(l) \(\xrightarrow{\Delta}\)
- NHivNO3(southward) \(\xrightarrow{\Delta}\)
- Sr(s) + N2(g) →
Answer
- NO(g) + HiiO(l) \(\xrightarrow{\Delta}\) no reaction
- NH4NO3(s) \(\xrightarrow{\Delta}\) Northward2O(g) + 2H2O(g)
- 3Sr(southward) + Ntwo(g) → Sr3N2(s)
Reactions and Compounds of the Heavier Pnicogens
Similar the heavier elements of group 14, the heavier pnicogens form catenated compounds that contain simply unmarried bonds, whose stability decreases rapidly as we become downwardly the group. For example, phosphorus exists as multiple allotropes, the near common of which is white phosphorus, which consists of P4 tetrahedra and behaves similar a typical nonmetal. As is typical of a molecular solid, white phosphorus is volatile, has a low melting betoken (44.1°C), and is soluble in nonpolar solvents. It is highly strained, with bond angles of only 60°, which partially explains why it is so reactive and and then easily converted to more stable allotropes. Heating white phosphorus for several days converts it to crimson phosphorus, a polymer that is air stable, about insoluble, denser than white phosphorus, and higher melting, properties that make it much safer to handle. A third allotrope of phosphorus, black phosphorus, is prepared by heating the other allotropes under loftier pressure; it is fifty-fifty less reactive, denser, and higher melting than red phosphorus. Equally expected from their structures, white phosphorus is an electrical insulator, and ruby-red and black phosphorus are semiconductors. The three heaviest pnicogens—arsenic, antimony, and bismuth—all have a metal luster, but they are brittle (non ductile) and relatively poor electrical conductors.
As in group xiv, the heavier group 15 elements form catenated compounds that comprise only single bonds, whose stability decreases as we go downwards the group.
The reactivity of the heavier pnicogens decreases equally we go downwards the column. Phosphorus is by far the virtually reactive of the pnicogens, forming binary compounds with every element in the periodic tabular array except antimony, bismuth, and the noble gases. Phosphorus reacts apace with O2, whereas arsenic burns in pure Oii if ignited, and antimony and bismuth react with O2 only when heated. None of the pnicogens reacts with nonoxidizing acids such equally aqueous HCl, but all dissolve in oxidizing acids such as HNO3. But bismuth behaves like a metal, dissolving in HNO3 to requite the hydrated Bi3 + cation.
The reactivity of the heavier group 15 elements decreases equally we go downwards the cavalcade.
The heavier pnicogens can utilize energetically attainable 3d, 4d, or 5d orbitals to form dsp3 or dtwosp3 hybrid orbitals for bonding. Consequently, these elements ofttimes accept coordination numbers of 5 or college. Phosphorus and arsenic form halides (eastward.thou., AsClv) that are by and large covalent molecular species and behave like typical nonmetal halides, reacting with water to form the corresponding oxoacids (in this case, H3AsOiv). All the pentahalides are strong Lewis acids that can aggrandize their coordination to accommodate the alone pair of a Lewis base:
\[\ce{AsF5(soln) + F^{−}(soln) \rightarrow AsF^{−}half dozen(soln)} \label{Eq4}\]
In dissimilarity, bismuth halides accept extended lattice structures and dissolve in h2o to produce hydrated ions, consistent with the stronger metallic character of bismuth.
Except for BiFiii, which is essentially an ionic compound, the trihalides are volatile covalent molecules with a alone pair of electrons on the key cantlet. Like the pentahalides, the trihalides react rapidly with water. In the cases of phosphorus and arsenic, the products are the respective acids, \(\ce{H3PO3}\) and \(\ce{H3AsO3}\), where Due east is P or As:
\[\ce{EX3(l) + 3H2O(l) \rightarrow H3EO3(aq) + 3HX(aq)} \label{Eq5}\]
Phosphorus halides are also used to produce insecticides, flame retardants, and plasticizers.
Phosphorus has the greatest power to form π bonds with elements such as O, N, and C.
With energetically accessible d orbitals, phosphorus and, to a lesser extent, arsenic are able to form π bonds with 2nd-period atoms such as Northward and O. This effect is fifty-fifty more important for phosphorus than for silicon, resulting in very strong P–O bonds and even stronger P=O bonds. The first iv elements in group 15 as well react with oxygen to produce the corresponding oxide in the +3 oxidation state. Of these oxides, P4Ohalf-dozen and As4O6 have cage structures formed by inserting an oxygen atom into each edge of the P4 or Equallyiv tetrahedron (function (a) in Figure \(\PageIndex{ii}\)), and they conduct like typical nonmetal oxides. For example, P4O6 reacts with water to form phosphorous acrid (HthreePO3). Consistent with its position between the nonmetal and metallic oxides, Sb4Ovi is amphoteric, dissolving in either acid or base. In contrast, Bi2O3 behaves similar a bones metallic oxide, dissolving in acrid to give solutions that comprise the hydrated Bi3 + ion. The ii least metallic elements of the heavier pnicogens, phosphorus and arsenic, course very stable oxides with the formula Due eastivO10 in the +v oxidation state (role (b) in Figure \(\PageIndex{two}\). In contrast, Bi2O5 is so unstable that in that location is no absolute proof it exists.
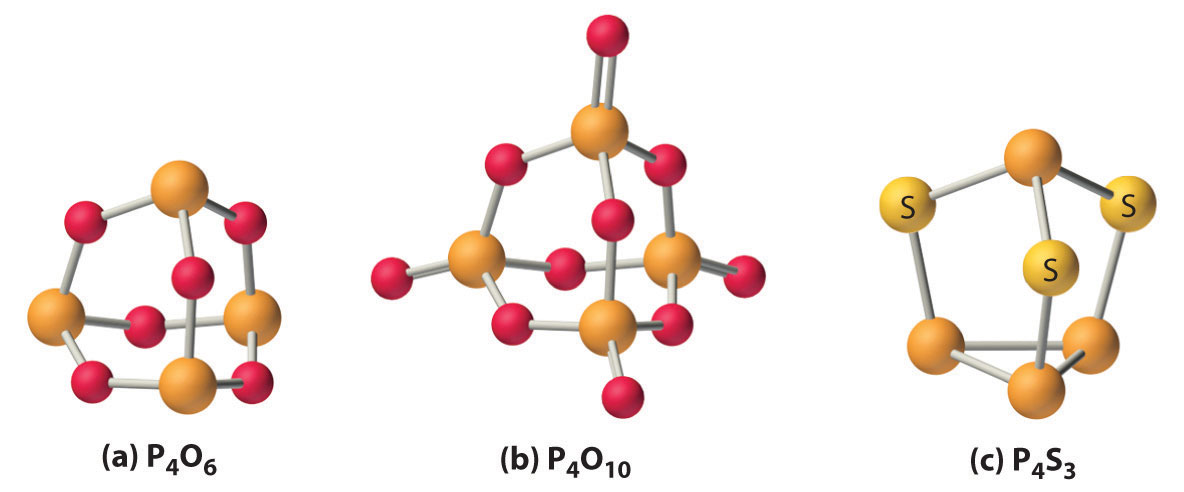
The heavier pnicogens form sulfides that range from molecular species with three-dimensional cage structures, such equally P4Southwardthree (office (c) in Effigy \(\PageIndex{2}\)), to layered or ribbon structures, such as Sb2Due south3 and BitwoSthree, which are semiconductors. Reacting the heavier pnicogens with metals produces substances whose backdrop vary with the metal content. Metal-rich phosphides (such as MfourP) are hard, loftier-melting, electrically conductive solids with a metallic luster, whereas phosphorus-rich phosphides (such as MP15) are lower melting and less thermally stable because they contain catenated Pn units. Many organic or organometallic compounds of the heavier pnicogens containing one to five alkyl or aryl groups are also known. Because of the decreasing strength of the pnicogen–carbon bond, their thermal stability decreases from phosphorus to bismuth.
The thermal stability of organic or organometallic compounds of group xv decreases down the group due to the decreasing strength of the pnicogen–carbon bond.
For each reaction, explain why the given products form.
- \(\mathrm{Bi(s) +\frac{3}{two}Br(50)\rightarrow BiBr_3(southward)}\)
- 2(CHiii)3As(l) + O2(g) → 2(CHiii)3As=O(due south)
- PBrthree(l) + 3HtwoO(l) → HiiiPO3(aq) + 3HBr(aq)
- As(due south) + Ga(due south) \(\xrightarrow{\Delta}\) GaAs(southward)
Given: balanced chemical equations
Asked for: why the given products form
Strategy:
Classify the blazon of reaction. Using periodic trends in diminutive backdrop, thermodynamics, and kinetics, explicate why the reaction products form.
Solution
- Bromine is an oxidant, and bismuth is a metallic that tin can be oxidized. Hence a redox reaction is likely to occur. To place the production, recall that bismuth tin can form compounds in either the +3 or +v oxidation state. The heaviest pnicogen, bismuth is rather hard to oxidize to the +5 oxidation land because of the inert-pair event. Hence the product will probably exist bismuth(III) bromide.
- Trimethylarsine, with a lone pair of electrons on the arsenic atom, can human action as either a Lewis base or a reductant. If arsenic is oxidized past two electrons, then oxygen must be reduced, well-nigh probably by two electrons to the −2 oxidation state. Because Every bit(V) forms strong bonds to oxygen due to π bonding, the expected production is (CH3)threeAs=O.
- Phosphorus tribromide is a typical nonmetal halide. We await it to react with water to produce an oxoacid of P(III) and the corresponding hydrohalic acid.Considering of the strength of the P=O bail, phosphorous acid (H3PO3) is really HP(O)(OH)2, which contains a P=O bond and a P–H bond.
- Gallium is a metal with a potent tendency to act every bit a reductant and class compounds in the +3 oxidation state. In contrast, arsenic is a semimetal. Information technology can human action as a reductant to form compounds in the +3 or +5 oxidation state, or information technology tin can act equally an oxidant, accepting electrons to form compounds in the −iii oxidation state. If a reaction occurs, so a binary compound will probably class with a 1:1 ratio of the elements. GaAs is an example of a III-V compound, many of which are used in the electronics manufacture.
Predict the products of each reaction and write a balanced chemic equation for each reaction.
- PCl5(s) + HtwoO(l) →
- BitwoO5(s) \(\xrightarrow{\Delta}\)
- Ca3Pii(southward) + H+(aq) →
- NaNH2(south) + PH3(soln) →
Answer
- PCl5(southward) + 4HtwoO(l) → H3PO4(aq) + 5HCl(aq)
- BitwoOfive(s) \(\xrightarrow{\Delta}\) Bi2O3(due south) + O2(g)
- CaiiiP2(s) + 6H+(aq) → 2PH3(g) + 3Ca2 +(aq)
- NaNHtwo(s) + PHthree(soln) → NaPH2(s) + NHthree(soln)
Summary
The reactivity of the heavier group 15 elements decreases downwardly the group, as does the stability of their catenated compounds. In group fifteen, nitrogen and phosphorus behave chemically like nonmetals, arsenic and antimony behave similar semimetals, and bismuth behaves like a metal. Nitrogen forms compounds in nine different oxidation states. The stability of the +5 oxidation state decreases from phosphorus to bismuth because of the inert-pair effect. Due to their higher electronegativity, the lighter pnicogens course compounds in the −3 oxidation state. Because of the presence of a solitary pair of electrons on the pnicogen, neutral covalent compounds of the trivalent pnicogens are Lewis bases. Nitrogen does not form stable catenated compounds because of repulsions between alone pairs of electrons on adjacent atoms, simply information technology does form multiple bonds with other 2d-period atoms. Nitrogen reacts with electropositive elements to produce solids that range from covalent to ionic in character. Reaction with electropositive metals produces ionic nitrides, reaction with less electropositive metals produces interstitial nitrides, and reaction with semimetals produces covalent nitrides. The reactivity of the pnicogens decreases with increasing atomic number. Compounds of the heavier pnicogens often have coordination numbers of 5 or college and use dspiii or d2sp3 hybrid orbitals for bonding. Because phosphorus and arsenic take energetically accessible d orbitals, these elements form π bonds with second-period atoms such as O and N. Phosphorus reacts with metals to produce phosphides. Metal-rich phosphides are hard, high-melting, electrically conductive solids with metallic luster, whereas phosphorus-rich phosphides, which contain catenated phosphorus units, are lower melting and less thermally stable.
A Semimetal In Group 5a,
Source: https://chem.libretexts.org/Bookshelves/General_Chemistry/Map%3A_Chemistry_(Zumdahl_and_Decoste)/18%3A_The_Representative_Elements/18.07%3A_The_Group_5A_Elements
Posted by: pooreexagavied.blogspot.com


0 Response to "A Semimetal In Group 5a"
Post a Comment